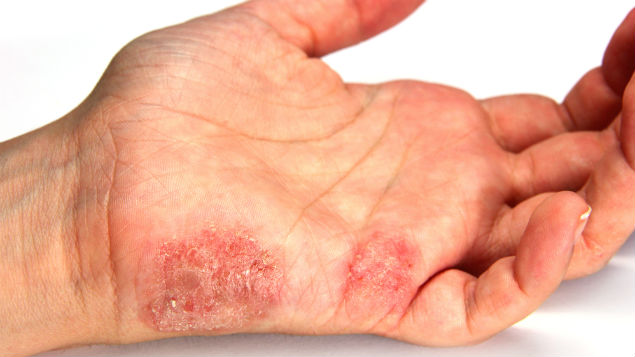
First non-animal allergy testing strategy approved
Submitted by:
Andrew Warmington
The Organisation for Economic Co-operation & Development (OECD) has approved the world’s first toxicology testing strategy without animal testing. This can now be used to test for skin sensitisation and any products that pass can be placed on the market.
The strategy, which consists of three ‘alternative’ methods, was jointly developed and validated over ten years by BASF and Givaudan, in partnership with various companies and scientific institutions, notably IIVS. It is claimed to have better predictivity for human allergy risks than traditional animal testing.
“In principle, this has opened up the possibility of approvals for entire testing strategies in other areas, such as eye irritation effects or effects on the hormone system,” BASF said.
In addition, the OECD has approved the Kinetic Direct Peptide Reactivity Assay, which also emerged from the work between BASF and Givaudan and measures the intensity of an allergic reaction is. This complements the approved strategy and can add information to it, thus entirely removing the need for animal tests for these reactions.
