WuXi AppTec triples peptide capacity
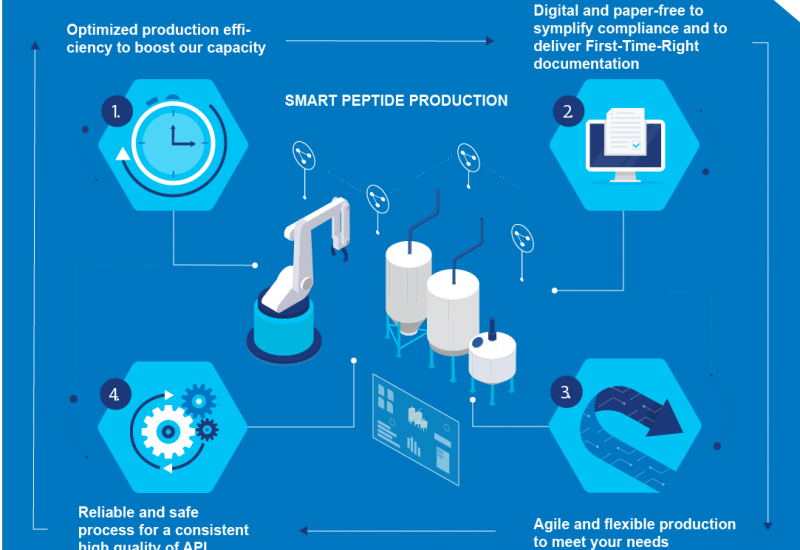
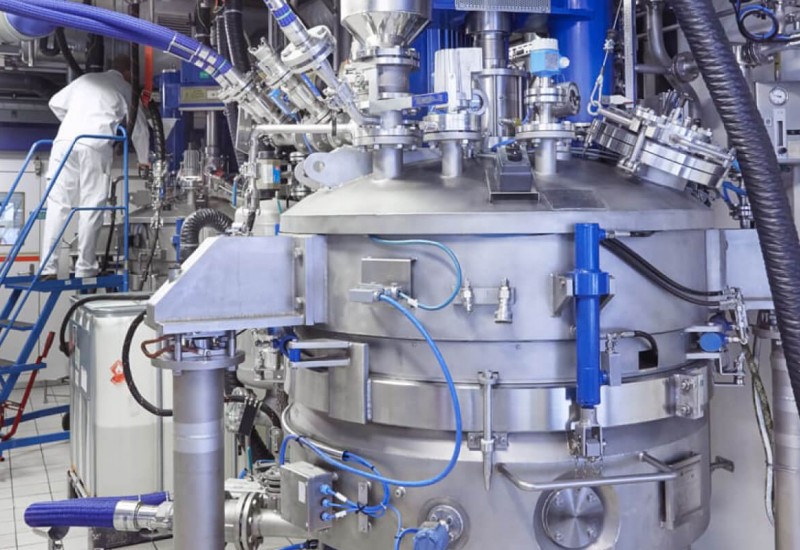
In response to growing demand worldwide, WuXi AppTec has commissioned two new peptide plants, one each at its Changzhou and Taixing sites in China. Both use digital operation systems with automated solvent delivery, in order to optimise production consistency, minimise human errors and reduce production cycle time.
This investment will increase the company’s total solid-phase peptide synthesis (SPPS) reactor volume to 32,000 litres. It also marks the official launch of Taixing as WuXi AppTec’s fifth API production sites.